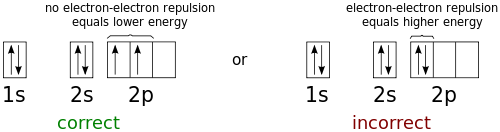
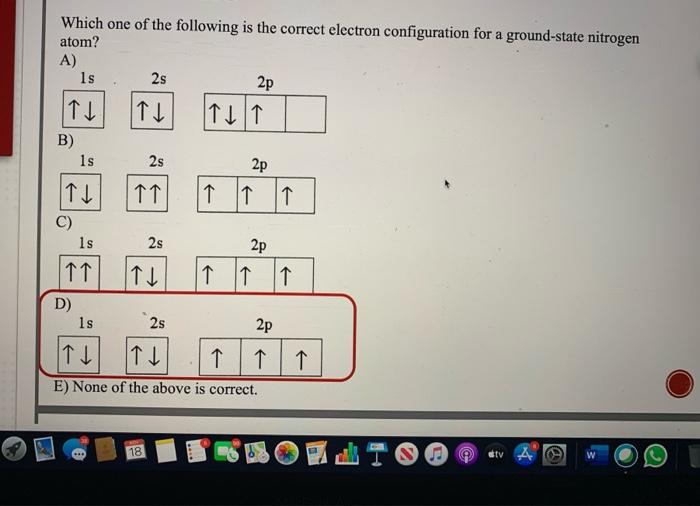
the orbital diagram for a ground state nitrogen atom is
It has one of the highest electronegativities among the elements 304 on the Pauling scale exceeded only by chlorine. Diamond is a solid form of pure carbon with its atoms arranged in a crystal.

Complete The Atomic Orbital Diagram For The Ground State Electronic Configuration Of Chlorine Homework Study Com
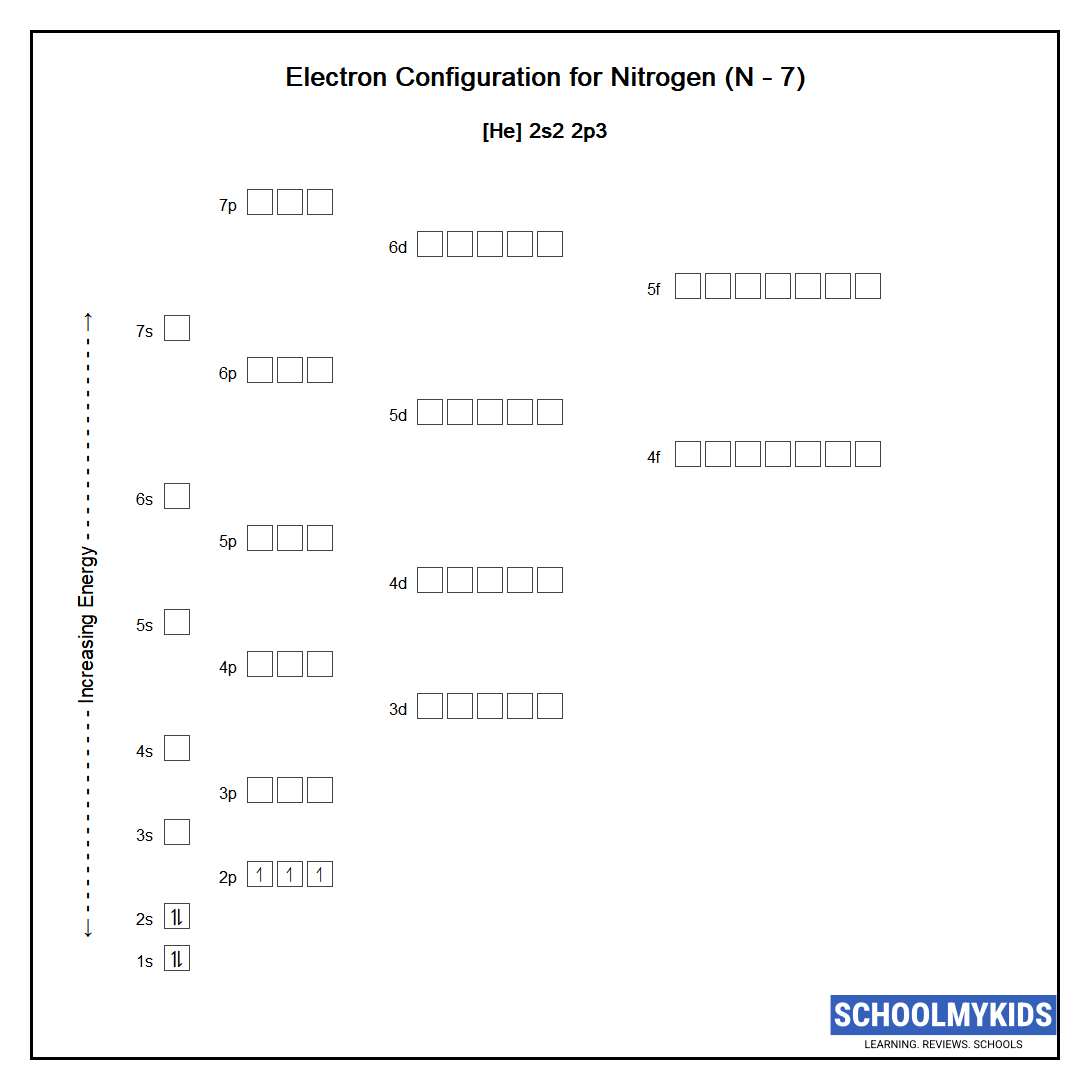
In the ground state they are arranged in the electron configuration 1s 2 2s 2 2p 1 x 2p 1 y 2p 1 zIt therefore has five valence electrons in the 2s and 2p orbitals three of which the p-electrons are unpaired.

. Every solid liquid gas and plasma is composed of neutral or ionized atoms. Mulliken in 1932 to mean. In the nitrogen ground-state electron configuration the three electrons of the 3p orbital are located in the p x p y and p z orbitals and the spin of the three electrons is the same.
In the air carbon dioxide is transparent to visible light but absorbs infrared radiation acting as a greenhouse gasIt is a trace gas in Earths atmosphere at 417. A liquid is a nearly incompressible fluid that conforms to the shape of its container but retains a nearly constant volume independent of pressure. Carbon from Latin carbo coal is a chemical element with the symbol C and atomic number 6.
It is nonmetallic and tetravalentits atom making four electrons available to form covalent chemical bondsIt belongs to group 14 of the periodic table. The formal charge is the charge a bonded atom in a molecule would have if the electrons in its bonding pairs were shared evenly. Solid carbon comes in different forms known as allotropes depending on the type of chemical bond.
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features. The ground state electron configuration of boron is 1s 2 2s 2 2p 1. An atom is the smallest unit of ordinary matter that forms a chemical element.
The ground-state electron configuration of fluorine is 1s 2 2s 2 2p 5. Orbital Diagram for Boron Electron configuration of boron in the excited state. Nitrogen atomic number 7 fills the 1s and 2s subshells and has one electron in each of the three 2p orbitals in accordance with Hunds rule.
Carbon dioxide chemical formula CO 2 is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. The electron configuration of all the elements can be done through the orbital diagram. Although more generally the rule is applicable for the s-block and p.
Browse our listings to find jobs in Germany for expats including jobs for English speakers or those in your native language. Atoms can jump from one orbital to another in an excited state. You fill in the order form with your basic requirements for a paper.
The terms atomic orbital and molecular orbital were introduced by Robert S. The orbitals are p x p y and p z and each orbital can have a maximum of two electrons. Atoms are extremely small typically around 100 picometers across.
The orbitals are 1s 2s 2p and 3s. The two most common allotropes of pure carbon are diamond and graphiteIn graphite the bonds are sp 2 orbital hybrids and the atoms form in planes with each bound to three nearest neighbors 120. The octet rule is a chemical rule of thumb that reflects the theory that main-group elements tend to bond in such a way that each atom has eight electrons in its valence shell giving it the same electronic configuration as a noble gasThe rule is especially applicable to carbon nitrogen oxygen and the halogens.
For main group elements the orbitals. Electron configuration of oxygen atom through orbital. A molecular orbital diagram or MO diagram is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals LCAO method in particular.
In chemistry a molecular orbital is a mathematical function describing the location and wave-like behavior of an electron in a moleculeThis function can be used to calculate chemical and physical properties such as the probability of finding an electron in any specific region. Here the nitrogen atom has three unpaired electrons. Your academic level paper type and format the number.
Atomic energy shells are subdivided into sub-energy levels. This implies that if two or more orbitals of equal. As such it is one of the four fundamental states of matter the others being solid gas and plasma and is the only state with a definite volume but no fixed shapeA liquid is made up of tiny vibrating particles of matter such as.
They are so small that accurately predicting their behavior using classical physics as if they were tennis balls for example is not possible due to quantum effects. We already know that the p-subshell has three orbitals. We already know that.
These three electrons have unpaired spins. Carbon makes up only about 0025 percent of Earths crust. Identifying the formal charge is useful because it can help predict.
Hunds rule of maximum multiplicity is a rule based on observation of atomic spectra which is used to predict the ground state of an atom or molecule with one or more open electronic shellsThe rule states that for a given electron configuration the lowest energy term is the one with the greatest value of spin multiplicity. Our custom writing service is a reliable solution on your academic journey that will always help you if your deadline is too tight. It is found in the gas state at room temperature.
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atomsThese electron pairs are known as shared pairs or bonding pairs and the stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding. An orbital diagram for a ground-state electron configuration of an Aluminum atom is shown below-. The Magnesium orbital diagram contains 2 electrons in the 1s orbital 2 electrons in the 2s orbital the six electrons in the 2p orbital and the remaining two electrons in the 3s orbital.
Outermost shell of electrons in a ground-state atom. For many molecules the sharing of electrons allows each atom to attain the. This is called quantum jump.
The electron configuration and orbital diagram for carbon are. The electron configuration of an element with an atomic number greater than 18 cannot be properly determined according to the Bohr atomic model. The Aluminum orbital diagram contains 2 electrons in the 1s orbital 2 electrons in the 2s orbital the six electrons in the 2p orbital the two electrons in the 3s orbital and the remaining one electron in the 3p orbital.
A nitrogen atom has seven electrons. Three isotopes occur naturally 12 C and 13 C being stable while 14 C. Then correct electron configuration of nitrogen in the ground state will be 1s 2 2s 2 2p x 1 2p y 1 2p z 1.
An atomic clock is a clock that measures time by monitoring the resonant frequency of atoms. It is based on atoms having different energy levelsElectron states in an atom are associated with different energy levels and in transitions between such states they interact with a very specific frequency of electromagnetic radiationThis phenomenon serves as the basis for the. An orbital diagram for a ground-state electron configuration of the Magnesium atom is shown below-.
The neutron is a uniquely useful tool for studying the microscopic magnetic. In chemistry orbital hybridisation or hybridization is the concept of mixing atomic orbitals to form new hybrid orbitals with different energies shapes etc than the component atomic orbitals suitable for the pairing of electrons to form chemical bonds in valence bond theoryFor example in a carbon atom which forms four single bonds the valence-shell s orbital combines. In the fluorine ground-state electron configuration the five electrons of the 3p orbital are located in the p x p y and p z.
A fundamental principle of these theories is that as atoms bond to form molecules a certain number of atomic orbitals combine to form the same. Spedding and his colleagues at Iowa State University began to produce large quantities of pure rare earth elements and to fashion them into single crystals and during the same period intense beams of thermal neutrons became available from research reactors.
High School Chemistry Orbital Configurations Wikibooks Open Books For An Open World
How Many Unpaired Electrons Are In An N2 Molecule Quora
How To Write The Orbital Diagram For Nitrogen N Youtube

Electron Diagrams And Excited State Youtube
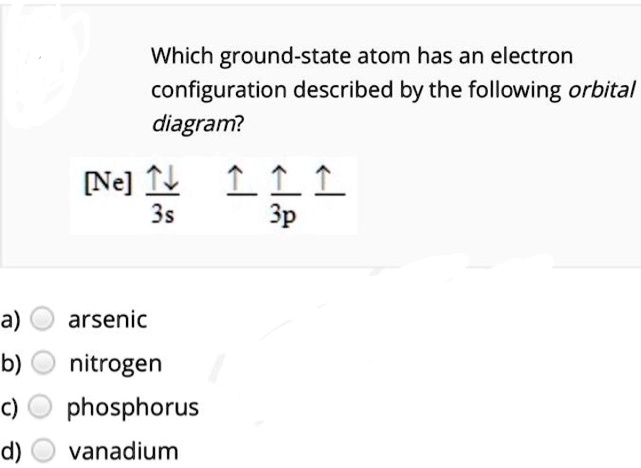
Solved Which Ground State Atom Has An Electron Configuration Described By The Following Orbital Diagram Nne 111 3s 3p A Arsenic B Nitrogen Phosphorus Vanadium C D

Solved Figure P7 7 7 8 Which Orbital Diagram In Figure P7 8 Chegg Com

Plan For Fri 7 Nov 08 Lecture Periodic Trends 7 12 Types Of Chemical Bonds 8 1 Electronegativity 8 2 Quiz Ppt Download

Write The Orbital Diagram For The Ground State Of The Arsenic Atom Give All Orbitals Homework Study Com
N Nitrogen Element Information Facts Properties Trends Uses And Comparison Periodic Table Of The Elements Schoolmykids
Solved Which One Of The Following Is The Correct Electron Chegg Com
File Orbital Diagram Nitrogen Svg Wikipedia

Exam 3 Study Guide Flashcards Quizlet

Orbital Filling Diagrams Wize University Chemistry 1 Textbook Wizeprep

Electron Configuration Worksheet Easy Hard Science

Answered Which Of The Orbital Diagrams Bartleby

Nitrogen Orbital Diagram Electron Configuration And Valence Electron
Solved These Two Pictures Are From The Same Question Course Hero